|
Researchers discovered that fat tissue releases
signals called microRNAs into the bloodstream that
regulate genes in another organ.
The findings suggest new ways to treat
metabolism-related diseases like obesity and
diabetes.
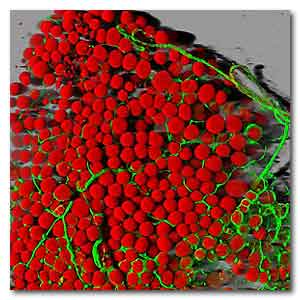
Mouse fat cells (red) and blood vessels (green).Daniela
Malide, NIH National Heart, Lung, and Blood
Institute.
Fat tissue releases hormones and other substances
that help regulate your body’s metabolism by
communicating with other organs and tissues, such as
your liver, pancreas, and muscles.
Several cell types, including fat cells, make small
pieces of genetic material, called microRNAs.
The precise roles of microRNAs are currently under
intense investigation.
High levels of certain microRNAs have shown to
correlate with the presence of several diseases,
including cancer, diabetes, heart disease, and
obesity.
The amount of microRNA in white fat tissue is known
to decline with age. This is due to lower levels of
an enzyme that processes microRNAs called Dicer.
To better understand the relationship between fat
cells, microRNA, and metabolism, a team led by Dr.
C. Ronald Kahn of the Joslin Diabetes Center and
Harvard Medical School looked at the effects of
removing Dicer from both white and brown fat cells
in mice. The study was supported in part by NIH’s
National Institute of Diabetes and Digestive and
Kidney Diseases (NIDDK). Results appeared on
February 23, 2017, in Nature.
Removing the Dicer gene from fat cells prevented the
cells from making microRNAs.
Animals missing the enzyme in their fat cells had
less white fat than control mice.
They also had brown fat with altered properties and
were insulin resistant.
The researchers measured microRNA levels in blood
samples taken from both normal and genetically
altered mice.
Most microRNAs are found in tiny, fluid-filled sacs
called exosomes.
Levels of hundreds of microRNAs were significantly
lower in the exosomes of mice lacking Dicer in their
fat. Of these, 88% were reduced by more than 4-fold.
MicroRNAs circulating in the blood outside of
exosomes were also lower in the mice lacking Dicer.
People with lipodystrophy—a condition characterized
by a lack of fat tissue—had low levels of
circulating exosomal microRNAs as well.
These results suggest that fat tissue is the main
source of circulating exosomal microRNAs in the
body.
The researchers transplanted fat from normal mice
into mice lacking Dicer in fat tissue. They found
that most microRNAs were restored to at least half
of their normal levels.
Brown fat transplants, but not white fat
transplants, also improved the animals’ glucose
metabolism.
The scientists next investigated whether microRNAs
released by fat tissue could affect other tissues.
Through a series of experiments, they showed that
circulating exosomal microRNAs from one mouse could
regulate gene expression in the liver of another.
These findings suggest that microRNAs made in fat
tissue can regulate metabolism and gene expression
throughout the body.
Because fat is easily accessible, it might offer a
way to deliver microRNAs to regulate genes in other
organs.
“This mechanism may offer the potential to develop
an entirely new therapeutic approach,” Kahn says.
For more information
U.S. National Institutes of Health
Link...
MDN |